Why does the risk of cancer increase as we age? As an 'inflammatory' it is the new tool to end the disease
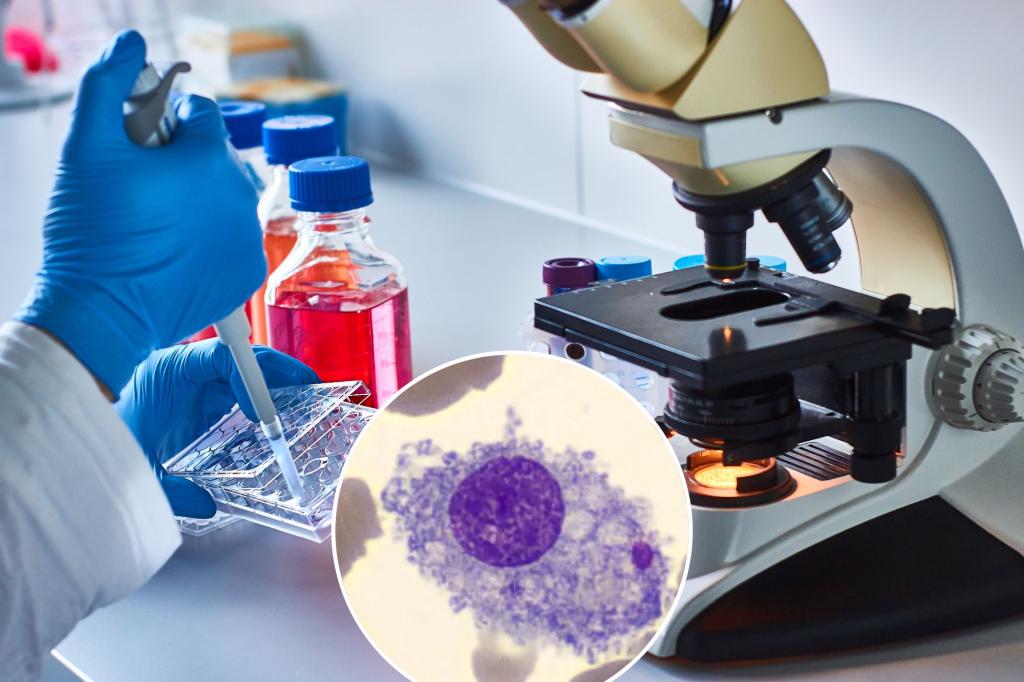
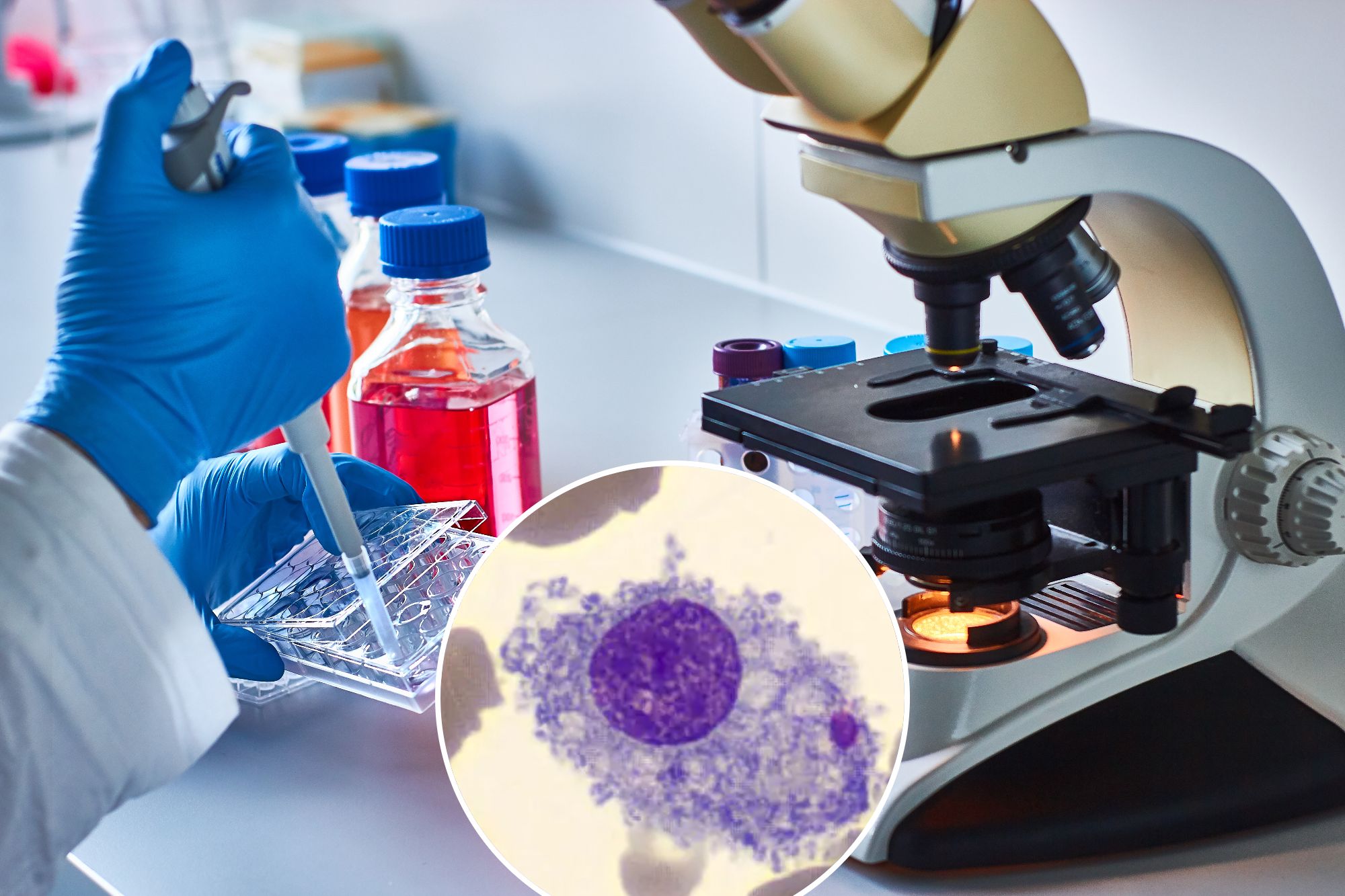
One of the great mysteries of cancer is why it primarily and almost exclusively targets people in middle age or older.
During the first five decades of life, you are twice as likely to die in a car accident than from cancer. But that suddenly changes at the half-century mark. "90% of cancers appear after age 50," says James DeGregori, Ph.D., associate director of the University of Colorado Cancer Center.
Why does our body suddenly stop being as effective at keeping cancer at bay after 50? There are theories—there's more accumulated cell damage, our immune systems lose some of their luster—but there's a new culprit that's fueled cancer research in recent decades: Inflammation.
Or rather, inflammatory, a "mash of inflammation and aging," says Dr. Brian Brown, director of the Icahn Genomics Institute at Mount Sinai in Manhattan. "It is a newly evaluated factor in the aging process that contributes to the higher likelihood of developing cancer in older people."
Everything science once believed about inflammation is changing, says Dr. Shilpa Ravella, an assistant professor of medicine at Columbia University Medical Center and author of A Silent Fire: The Story of Inflammation, Diet, and Disease (WW Norton, out now).
"The relationship between inflammation and cancer is more complex than previously imagined," she tells the Post. "Inflammation is, in fact, one of the "hallmarks" of cancer."
Inflammation, at its core, is a good thing. It's part of the body's immune response and the reason we don't die every time we get an infection. "In a healthy situation, your immune system fights the infection, eliminates the cancer cells, and the job is done," says Brown. "And then it closes itself."
But this changes with age. "Especially after age 40, our bodies have a harder time taming inflammation," says Brown. "That's why the elderly got sick a lot more from COVID. It was not the virus that killed many old people. It was the inflammation caused by the virus. They can't turn it off.”
Inflammation, or chronic inflammation, keeps the immune system on high alert "so it can't recognize and attack cancer cells as efficiently," says Stephen Perrine, author of the new book The Full-Body Fat Fix: The Science-Based 7-Day Plan to Calm Inflammation, Heal Your Gut, and Build a Healthier, Leaner You!” (St. Martin's Press) "It's like a fire company that has to keep responding to false alarms."
White blood cells continue to release cytokines, which leads to a constant state of inflammation, making it harder for the immune system to detect and attack cancer cells.
It is still a relatively new theory, and still controversial according to Dr. Ian Neel, an associate professor of medicine at the University of California, San Diego. “Is inflammation a marker of chronic disease, or does it cause chronic disease?” hey asks "We've found many correlations through research on inflammation and various disease states, but these don't always translate to causation." In other words, the link between chronic inflammation and cancer is still open to debate.
But Ravella claims this thinking is nothing less than "the biggest paradigm shift in the last two decades." Increasingly, she says, a growing body of research has shown that inflammation, once thought to be just a consequence of disease, may actually be an independent cause of chronic diseases like cancer.
"The relationship between inflammation and cancer is bidirectional," she tells the Post. "Inflammation drives cancer, and cancer drives more inflammation." Whether inflammation is present in the body before or after a cancer diagnosis, it affects all stages of the cancer life cycle—part of what Ravella calls the "tumor microenvironment"—"from the early genetic changes that transform normal cells into malignant ones to the continuous growth and spread of cancerous tissue throughout the body," she says.
Even low levels of inflammation, which doctors don't usually test for, can promote cancer, Ravella says.
So what to do about it? In the past, doctors have tried to treat diseases by completely shutting down inflammation. But that's a bit like trying to fix police corruption by firing the entire police department. "Inflammation is part of a normal immune process," says Brown. "Eliminating it would likely mean immunosuppressing someone, which we don't want to do because we need inflammatory processes to fight infections and even cancer. What we want to do is simply dampen overactive inflammation.”
Some of this can be done through food, such as the "anti-inflammatory" diets endorsed by Perrine, which include cutting out ultra-processed—or as he calls them, "predigested"—foods that promote inflammation and eating a mix of diverse. plant foods. "The wider the diversity of plants in your diet — at least 30 different types per week — the healthier your microbiome and the lower the inflammation," Perrine tells the Post.
But diet alone isn't enough, Brown says. "You can't eat away at genetic predispositions and environmental triggers that contribute to inflammation." Although it's widely claimed that all inflammation starts in the gut, inflammation can "start in any tissue," says Brown. "Your lungs getting infected with a virus can cause inflammation there. Cholesterol that builds up in the arteries can cause inflammation in the blood vessels. A tumor growing in an organ like the pancreas will cause inflammation.”
A better solution is a healthy diet along with the right medicine. Brown points to statins and LDL cholesterol (or "bad" cholesterol). "You can remove all sources of LDL cholesterol from your diet, but your body can still make LDL," he says. "So taking a statin has benefits beyond diet."
Appropriate drugs to fight inflammation are still being investigated. There is currently ongoing evidence for everything from silibinin – a milk thistle extract with anti-inflammatory properties being explored by Italian researchers – to metformin, a type 2 diabetes drug that may lower the risk of some gastrointestinal cancers .
Rapamycin, a drug commonly used in organ transplant care, has had encouraging results in experiments by German researchers, extending the lifespan of laboratory mice. But Dudley Lamming, an associate professor of medicine at the University of Wisconsin-Madison who conducted his own research on rapamycin, tells The Post it's too early to get excited.
"Suppressing the normal function of the immune system, which normally patrols for cancer, can actually cause a INCREASES at risk of cancer", he says. "There are increasing numbers of people taking rapamycin 'off label' for anti-aging purposes, but there is still no evidence that rapamycin can prolong healthy aging in humans."
One of the most promising research involves macrophages, the white blood cell "first responders" that are nature's best defense against cancer—and also, in the case of inflammation, the body "And you, Brute?" traitor.
Brown tells the Post that macrophages have been key to discovering the link between inflammation and cancer. Macrophages, and their precursors, monocytes, are "one of the most important types of immune cells for clearing infections," he says. "But they are also a key mediator of inflammation."
Recent studies have found that cancers not only outgrow macrophages, but also them recruit them, producing chemicals that cause macrophages to suppress inflammation and promote tumor growth. "In fact, some cancers have more macrophages than cancer cells," says Brown. Finding ways to control and redirect macrophages will have "a profound impact on cancer and potentially aging," he says.
Dr. Yara Abdou, an assistant professor of oncology at the University of North Carolina, has tried to do just that. In ongoing research with her colleague, Dr. Michael Klichinsky, she is exploring ways to "trick macrophages into thinking cancer cells are foreign invaders," she tells The Post.
They do this by inserting receptors designed to recognize specific cancer targets. When the engineered macrophages come into contact with the cancer cell, "they react the same way they would when they come into contact with a bacterium," says Abdou. “They eat the cancer cell, kill it and induce inflammation in the tumor – in this case, a very good thing. We use a patient's own immune cells to attack their cancer.”
Although their research is still in early Phase I trials, the results have been "very promising," says Abdou. "We have shown that we can engineer cells from patients with very advanced cancer. We have seen some lesions shrink after a single dose of CAR macrophages.” And while their research has focused on patients with breast, ovarian and esophageal cancer, it could be expanded to include other cancers. "One of the beautiful aspects of this technology is that it's a plug-and-play system," says Abdou.
Samuel Waxman Cancer Research Foundation
Cancer is just the beginning. "A clear link has emerged between aging and inflammation," says Brown. And discovering how inflammation plays a role in disease will not only lead to a cure for cancer, but could cure heart disease, Alzheimer's and arthritis.
"There is an urgency to understand why we become hyper-inflammatory as we age and how we can safely reduce this inflammation without causing immune suppression," says Brown. "We're getting closer to solving that, and it's going to help us live healthier lives."
#risk #cancer #increase #age #inflammatory #tool #disease
Image Source : nypost.com

Deja una respuesta